Information about the use of saliva samples for SARS-CoV-2 RNA detection
It has been demonstrated by various international and E. Gulbja Laboratorija studies, as well as described in the guidelines of the European Centre for Disease Prevention and Control, that saliva samples can be used to detect the presence of SARS-CoV-2 virus RNA in humans.
One of the main advantages of a saliva sample is the ease of obtaining the material, which can be performed by the patient him- or herself, and which does not cause discomfort during the sample collection process. Because of the fact that the collection of the sample does not require specially trained medical personnel, this can significantly expand the range of patients that can be tested.
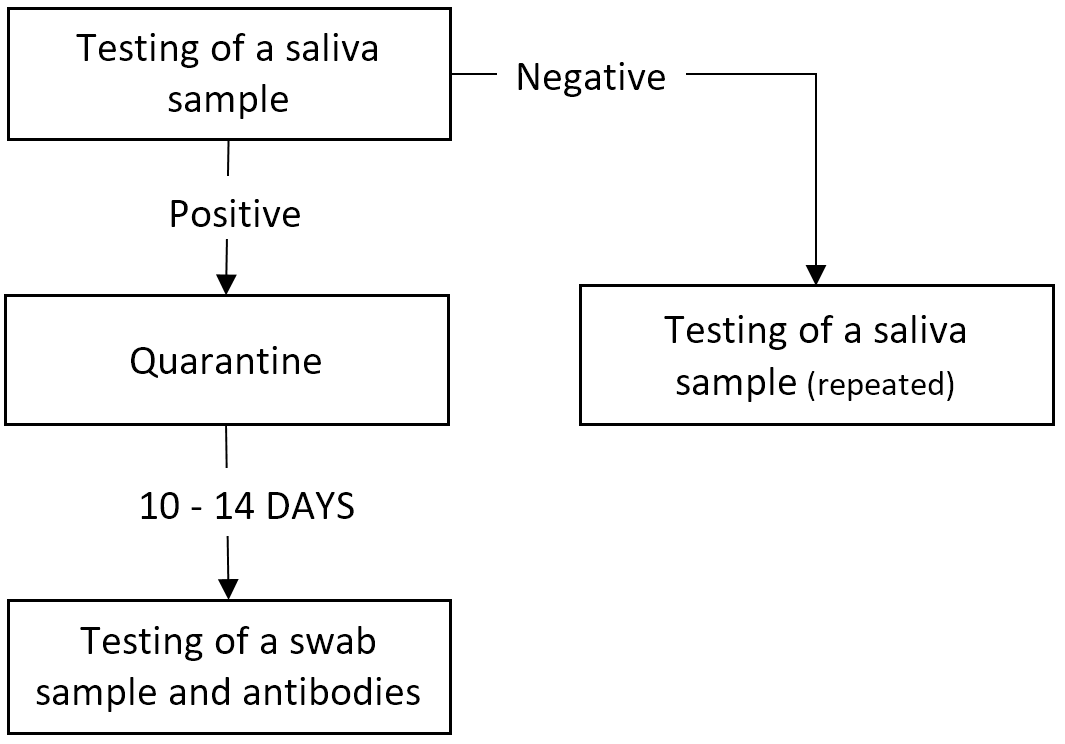
Detection of the virus in a saliva sample is intended for screening purposes, i.e., to find infected and/or infectious individuals in a group, and is to be performed on a regular basis, for example in a work group environment, in an educational establishment or in an outbreak area.
It should be noted that saliva samples cannot be used in all cases (both clinical and epidemiological).
Saliva samples should be used in the acute phase of the SARS-CoV-2 viral infection until approximately day 8 after the onset of clinical symptoms, and should NOT be used for the purposes of quarantine termination or other control testing in the following cases:
- The patient has previously had a positive SARS-CoV-2 viral RNA result from an oropharyngeal/nasopharyngeal swab specimen
- The patient has previously had a positive SARS-CoV-2 viral RNA result in a saliva sample
- The patient’s blood sample contains specific antibodies to the SARS-CoV-2 virus
E. Gulbja Laboratorija does not test samples like this.
An oropharyngeal and nasopharyngeal swab specimen should be used to terminate quarantine following COVID-19 disease or other control testing. If control testing is performed no earlier than day 14, it is recommended that specific SARS-CoV-2 virus antibodies be detected in the patient’s blood sample.
Saliva collection kits for medical practices are/will be delivered in coordination with the Latvian Centre for Disease Prevention and Control.